Crystal engineering with 1-benzofuran-2,3-dicarboxylic acid: co-crystals with bipyridyl ligands, discrete complexes and coordination polymers with metal ions†
Abstract
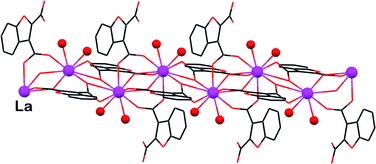
This study evaluates the versatile supramolecular reactivity of the title dicarboxylic ligand (

 Please wait while we load your content...
Please wait while we load your content...