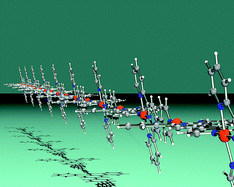
We have demonstrated that treatment of [Fe(1)2]2+ (1 = 4′-(4-pyridyl)-2,2′:6′,2″-terpyridine) with an excess of CuCl2·2H2O leads to competition between coordination of copper(II) by the pendant pyridine donors in [Fe(1)2]2+, and transfer of ligand 1 from iron(II) to copper(II) to yield [{Cl3Cu(µ-1)Fe(µ-1)}2CuCl2(OH2)2]Cl2·4H2O and [Cu(H1)Cl2]Cl·4H2O, respectively. Direct reaction of 1 with CuCl2·2H2O and variation in crystallization conditions results in the formation of either monomeric or polymeric complexes: [Cu(1)Cl2]·4.75H2O, [Cu(1)Cl2]·H2O·MeOH and [Cu(1)Cl2·2H2O]n. The reaction of 1 with Cu(NO3)2·3H2O gives the coordination polymer [Cu(1)(ONO2)2·H2O]n. Magnetic data for [Cu(1)Cl2·2H2O]n and [Cu(1)(ONO2)2·H2O]n are consistent with antiferromagnetically-coupled chains. Reactions of CuCl2·2H2O with ligands 2 and 3 (2 = 4′-(3-pyridyl)-2,2′:6′,2″-terpyridine, 3 = 4′-(2-pyridyl)-2,2′:6′,2″-terpyridine) lead to monomeric complexes. With the exception of [Cu(3)Cl2], the mononuclear complexes show a propensity for the incorporation of water into their crystal lattices; in the case of [Cu(H1)Cl2]Cl·4H2O and [Cu(H1)Cl2]Cl·4.75H2O, the small change in water content leads to the assembly of (5.82)2(5.6.8)4(52.6)2 or (42.62)3(4.62)6water–chloride ion nets.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...