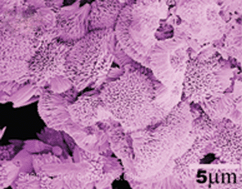
In this paper, flowerlike Co11(HPO3)8(OH)6 superstructures were selectively synthesized via a facile hydrothermal route on a large scale in the presence of a certain amount of Ni2+ ions, employing CoCl2·6H2O and NaH2PO2·H2O as the reactants, NaHCO3 as the pH adjustor. HPO3− ions were provided based on the dismutation of H2PO2− ions in a weak basic solution. The reaction was carried out at 170 °C for 10 h. The as-obtained products were characterized by X-ray powder diffraction (XRD), energy dispersive spectrometry (EDS), field emission scanning electron microscopy (SEM), selected area electron diffraction (SAED) and high resolution transmission electron microscopy (HRTEM). Research showed that the Ni2+ ions in the system played a crucial role in the formation of flowerlike Co11(HPO3)8(OH)6 superstructures. Some factors influencing the morphology of Co11(HPO3)8(OH)6, including the reaction time, temperature, and the kinds of metal ions were investigated. A possible growth mechanism was proposed.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...