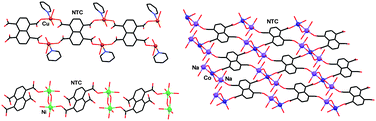
This study evaluates the supramolecular reactivity of the title tetracarboxylic ligand (NTCA), targeting the synthesis of hybrid organic–inorganic polymeric assemblies with the aid of metal–ligand coordination synthons. In the experimental conditions used, all reactions of the corresponding 2+ metal ions with NTCA were associated with a complete de-protonation of this ligand to its tetra-anionic form, naphthalene-1,4,5,8-tetracarboxylate (NTC), and a 2 : 1 metal : NTC stoichiometry in the resulting materials. The latter represent either hybrid coordination polymers of diverse and a priori unpredictable connectivity schemes (with CuII, NiII and CoII as connectors), or discrete NTC complexes with the nickel and cobalt ions. The formed polymeric arrays are of 1D or 2D nature. Based on the empirical observations, it appears that due to the high affinity of these transition metal ions for competing ligands from the solvent (e.g., water and pyridine) and the biased uni-directional disposition of the carboxylate groups on the molecular framework of NTC, the potential utility of the latter in a designed construction of spatially extended coordination frameworks is not very promising.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...