Synthesis, structure, photoluminescence and magnetic properties of new 3-D lanthanide-pyridine-2,4,6-tricarboxylate frameworks†
Abstract
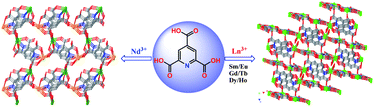
Seven 3-D (3D) metal–organic framework solids [Nd2(pyta)2(H2O)6]n1 and {[Ln2(pyta)2(H2O)x]·yH2O}n (Ln = Sm 2, Eu 3, Gd 4 with x = 3, y = 2.5; Ln = Tb 5 with x = 2, y = 2.5, Ln = Dy 6, Ho 7 with x = 2, y = 2), H3pyta = 2,4,6-pyridinecarboxylic acid) have been prepared under hydrothermal conditions. X-Ray structural analysis shows that compound 1 crystallises in monoclinic space groupPc and all Ln(III) ions are nine-coordinated. The 3D framework of 1 is constructed from [Nd4(µ4-pyta)2]n chains with 1D small channels along the a axis. Isomorphous compounds 2–7 crystallise in C2/c space group and are composed of alternative [Ln4(µ5-pyta)2]n chains and [Ln6(µ4-pyta)2]n helical chains. The completely deprotonated pyta3−

 Please wait while we load your content...
Please wait while we load your content...