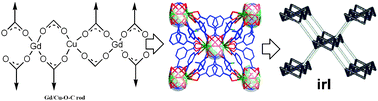
A new member of 3d-4f metal–organic framework (MOF), Gd2Cu(μ-H2O)2(ip)4(CH3CH(NH3)CO2)2·H2O (1, ip = 1,3-benzene dicarboxylate), was generated by the hydrothermal self-assembly of Gd2O3, CuCl2, H2ip, and L-alanine. In 1, the slightly distorted CuO4 squares combine with GdO9 polyhedra via ip and CH3CH(NH3)CO2 carboxyl groups to create the Gd2Cu(CO2)8(CH3CH(NH3)CO2)2 rod-like substructure; such species are further linked together by phenyl units of ip to give the pcu-type rod packing. The subsequent topology analysis based on O'Keeffe's definition and classification of rod-based MOFs suggests the irl net of 1. Moreover, as evidenced by TG and XRD research, the potential microporous structure that owns the solvent-accessible volume of 246 Å3, equal to 5.7% of the cell volume, can be maintained until 285 °C after the loss of coordinated water molecules. The magnetic measurements suggest weak ferromagnetic interactions within the Gd2Cu(CO2)8(CH3CH(NH3)CO2)2 rod-like substructure, which is estimated by the fitting of χM = C/(T-θ) with θ = +0.2K and a simplified Gd–J–Cu–J–Gd mode with J = +0.23 cm−1, g = 2.0, TIP = 100 × 10−6 cm3 mol−1K.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...