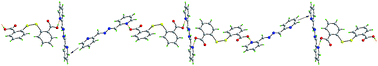
The structure of the 1 : 1 co-crystal formed between the dicarboxylic acid, 2,2′-dithiodibenzoic acid, and each of the isomeric 3- and 4-pyridinealdazines features a supramolecular zig-zag chain constructed about the O–Hacid⋯Npyridine synthon. By contrast, using the same experimental conditions, only a 2 : 3 co-crystal could be formed when the isomeric 2-pyridinealdazine was used. Here, a pentamer is found, stabilised by the O–Hacid⋯Npyridine synthon and these associate to form a supramolecular chain via C–H⋯π contacts. Theory shows no significant differences in the basicity of the pyridine-nitrogen atoms leading to the conclusion that it is the steric congestion related to the relative disposition of the 2-pyridine nitrogen that is responsible for the observed behaviour.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?

 Please wait while we load your content...
Please wait while we load your content...