Preferential crystallization (AS3PC mode) of modafinic acid: an example of productivity enhancement by addition of a non-chiral base†
Abstract
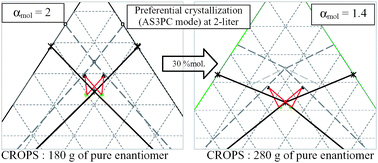
Modafinic acid belongs to the minor fraction of chiral components whose racemic mixture crystallizes as a stable conglomerate. Preferential

 Please wait while we load your content...
Please wait while we load your content...