Abstract
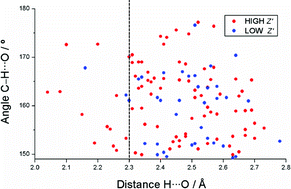
The higher Z′ polymorph has a shorter C–H⋯O interaction in 26 out of 38 crystal structures with two or more forms; these data suggest the dominance of stronger hydrogen bonds, or enthalpy, in the crystallization of multiple Z′ crystal structures.

 Please wait while we load your content...
Please wait while we load your content...