Synthesis and structure of 2-acetylpyridine-salicyloylhydrazone and its copper(ii) and zinc(ii) complexes. The effect of the metal coordination on the weak intermolecular interactions
Abstract
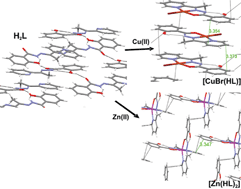
We have studied the crystal and molecular structure of 2-acetylpyridine-salicyloylhydrazone (H2L) and its copper and zinc complexes. The crystal structure of H2L is mainly dominated by the intermolecular moderate hydrogen bonding O(2)–H⋯O(1) whereas the intramolecular interaction N(1)–H⋯O(2) is responsible for the almost planar molecular structure. Reaction with copper and zinc acetates afford the complexes [CuBr(HL)] and [Zn(HL)2]. The

 Please wait while we load your content...
Please wait while we load your content...