Three concomitant polymorphs of 1 : 1 4,4′-dihydroxybenzophenone/1,2-bis(4-pyridyl)-ethylene: applications of hydrothermal method in searching polymorphs
Abstract
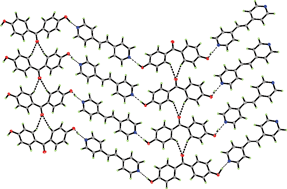
The cocrystallization of 4,4′-dihydroxybenzophenone and 1,2-bis(4-pyridyl)ethylene under hydrothermal condition provides 1 : 1 adduct, which has been found to present three polymorphs 1, 2 and 3 with space group P2/c, Pca21 and C2/c, respectively. 4,4′-Dihydroxybenzophenone in these three compounds adopts different conformations, but they have a similar layer structure, in which 4,4′-dihydroxybenzophenone molecules are connected by 1,2-bis(4-pyridyl)-ethylene into a 1-D chain through O–H⋯N hydrogen bonds and C–H⋯O weak hydrogen bonds between adjacent 4,4′-dihydroxybenzophenone from neighboring chains further link these chains into a 2-D layer. Compound 3 has higher density than 1 and 2 due to significant π–π interactions between phenyl rings from adjacent layers.

 Please wait while we load your content...
Please wait while we load your content...