Effect of the steric demand and hydrogen bonding capability of additives on the crystal polymorphism of sulfathiazole
Abstract
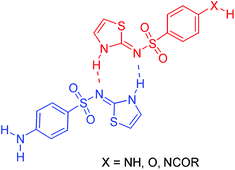
The N-acylsulfathiazole deriviatives 2, 3 and 4, the polymeric N-acylsulfathiazole 6 and the phenolic sulfathiazole analogue 7 were prepared and were found to promote

 Please wait while we load your content...
Please wait while we load your content...