Stereoselective copper-catalyzed heteroarene C–H functionalization/Michael-type annulation cascade with α-diazocarbonyls†
Abstract
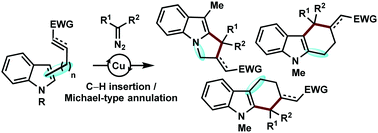
A stereoselective, copper-catalyzed, arene C(sp2)–H functionalization/Michael-type annulation reaction involving α-diazocarbonyl compounds has been developed. The method features low catalyst loadings, high yields, and excellent regio and stereoselectivity, in the synthesis of various heteroaromatic frameworks by employing indoles as the arene partner.


 Please wait while we load your content...
Please wait while we load your content...