Electrochemical trifluoromethylation/cyclization for the synthesis of isoquinoline-1,3-diones and oxindoles†
Abstract
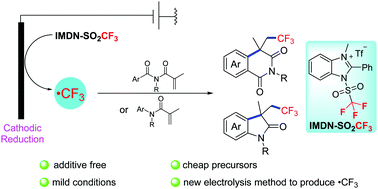
Herein, we describe a protocol for electrochemical cathode reduction to generate trifluoromethyl radicals. The trifluoromethylation reagent (IMDN-SO2CF3) used in this strategy is inexpensive and easy to obtain, and the reaction can be conducted efficiently without the addition of additional redox reagents. Using this strategy, we achieved electrochemical trifluoromethylation/cyclization for the synthesis of isoquinoline-1,3-diones and oxindoles. This protocol has good functional group tolerance and a broad substrate scope.
- This article is part of the themed collection: Green Synthesis


 Please wait while we load your content...
Please wait while we load your content...