Metal-free C8–H functionalization of quinoline N-oxides with ynamides†
Abstract
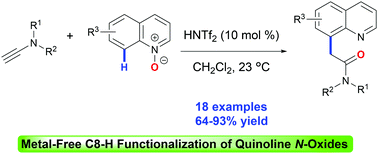
The metal-free C8–H functionalization of quinoline N-oxides with ynamides is unveiled for the first time by the intramolecular Friedel–Crafts-type reaction of quinolyl enolonium intermediates generated from Brønsted acid-catalyzed addition of quinoline N-oxides to ynamides. Various quinoline N-oxides and terminal ynamides prove to be suitable substrates for this method. A one-pot protocol was then developed for the metal-free direct C8–H functionalization of quinolines.


 Please wait while we load your content...
Please wait while we load your content...