Intermolecular trifluoromethyl-alkenylation of alkenes enabled by metal-free photoredox catalysis†
Abstract
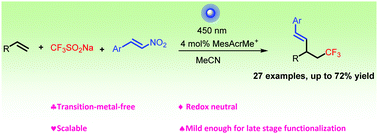
A three-component and redox-neutral trifluoromethylative alkenylation of unactivated alkenes with β-nitrostyrenes has been developed under visible-light. This metal-free protocol utilizes the easy to handle Langlois reagent (CF3SO2Na) as the CF3 source and is suitable for various unactivated alkenes and β-nitrostyrenes, affording a series of trifluoromethylated aromatic alkenes under mild conditions in good to excellent yields.


 Please wait while we load your content...
Please wait while we load your content...