Base promoted gem-difluoroolefination of alkyl triflones†
Abstract
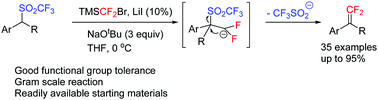
A new synthesis of gem-difluoroalkenes from readily available alkyl triflones and difluorocarbene precursors such as TMSCF2Br has been reported. The reaction, regardless of electronic effect, gives gem-difluoroalkenes in good to excellent yields. The mechanism may involve deprotonation of triflones, nucleophilic addition, and the elimination of SO2CF3.


 Please wait while we load your content...
Please wait while we load your content...