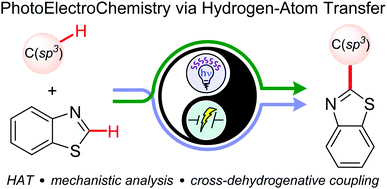
Photoelectrochemical cross-dehydrogenative coupling of benzothiazoles with strong aliphatic C–H bonds†
Abstract
A photoelectrochemical strategy for the cross-dehydrogenative coupling of unactivated aliphatic hydrogen donors (e.g. alkanes) with benzothiazoles is reported. We used tetrabutylammonium decatungstate as the photocatalyst to activate strong C(sp3)–H bonds in the chosen substrates, while electrochemistry scavenged the extra electrons.

 Please wait while we load your content...
Please wait while we load your content...