Three dimensional isophlorinoid tetrapodal molecular cage†
Abstract
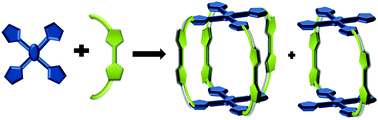
Steric hindrance induced by thiophene molecules in predesigned precursors favors the exclusive formation of a three dimensional (3D) π-conjugated cage and quasi-cage like molecules instead of a porphyrinoid macrocycle. Herein we report the synthesis of a tetrapod 3D fully π-conjugated molecular cage using a simple acid catalysed reaction. The X-Ray crystallography analysis confirmed the tetrapod cage structure and intermediates, which resemble three-fourths or half of the cage structures.


 Please wait while we load your content...
Please wait while we load your content...