Towards Cs-ion supercapacitors: Cs intercalation in polymorph MoS2 as a model 2D electrode material†
Abstract
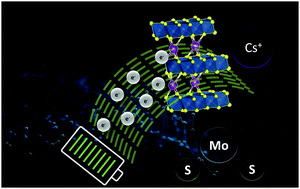
Intercalation of alkali metals has proved to be an effective approach for the enhancement of the energy storage performance in layered-2D materials. However, the research so far has been limited to Li and Na ion intercalation with K ions being recently investigated. Although cesium (Cs) salts are highly soluble in water, Cs+ intercalation has been addressed neither in batteries nor in supercapacitors so far. Herein, we demonstrate the exceptional effect of Cs+ intercalation in MoS2 as a model 2D material to boost its performance as a potential supercapacitor electrode. Cs+ intercalation was found to stabilize the metastable 1T phase and increase the conductivity of the 2H and 3R phases. Cs+-Intercalated 1T MoS2 showed higher quantum capacitance (CQ) than doped graphene.


 Please wait while we load your content...
Please wait while we load your content...