Catalytic asymmetric oxidative carbonylation-induced kinetic resolution of sterically hindered benzylamines to chiral isoindolinones†
Abstract
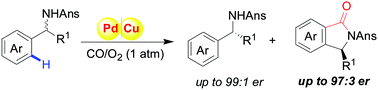
A highly enantioselective kinetic resolution of sterically hindered benzylamines has been achieved for the first time through transition-metal-catalyzed oxidative carbonylation, in which the new KR strategy offered a new approach to afford chiral isoindolinones (er up to 97 : 3) and the origin of chemoselectivity and stereoselectivity was confirmed by density functional theory (DFT) calculations.


 Please wait while we load your content...
Please wait while we load your content...