Design of oxa-spirocyclic PHOX ligands for the asymmetric synthesis of lorcaserin via iridium-catalyzed asymmetric hydrogenation†
Abstract
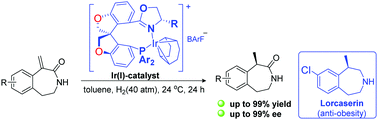
Phosphine–oxazoline (PHOX) ligands are a very important class of privileged ligands in asymmetric catalysis. A series of highly rigid oxa-spiro phosphine–oxazoline (O-SIPHOX) ligands based on O-SPINOL was synthesized efficiently, and their iridium complexes were synthesized by coordination of the O-SIPHOX ligands to [Ir(cod)Cl]2 in the presence of sodium tetrakis-3,5-bis(trifluoromethyl)phenylborate (NaBArF). The cationic iridium complexes showed high reactivity and excellent enantioselectivity in the asymmetric hydrogenation of 1-methylene-tetrahydro-benzo[d]azepin-2-ones (up to 99% yield and up to 99% ee). A key intermediate of the anti-obesity drug lorcaserin could be efficiently synthesized using this protocol.


 Please wait while we load your content...
Please wait while we load your content...