Direct sulfonylation of BODIPY dyes with sodium sulfinates through oxidative radical hydrogen substitution at the α-position†
Abstract
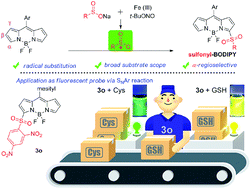
An efficient and convenient protocol for the direct sulfonylation of BODIPY dyes with sodium sulfinates via a radical process is described for the first time. This transformation presented wide substrate scope and high regioselectivity, providing a series of α-sulfonylated BODIPYs. Meaningfully, the sulfonyl group, as a good leaving group, allowed the facile introduction of a variety of functionalities on the BODIPY core. Moreover, a 2,4-dinitrobenzenesulfonyl (DBS) group substituted BODIPY showed dramatically quenched fluorescence via the photoinduced electron transfer (PET) pathway, and was demonstrated as a new fluorescent probe for selective biothiol detection.


 Please wait while we load your content...
Please wait while we load your content...