Triazole formation of phosphinyl alkynes with azides through transient protection of phosphine by copper†
Abstract
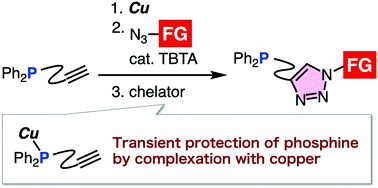
An efficient preparation method of functionalized phosphines by copper-catalyzed azide–alkyne cycloaddition (CuAAC) through the transient protection of phosphine from the Staudinger reaction is disclosed. Diverse phosphines were prepared from phosphinyl alkynes and azides by the click reaction at the ethynyl group without damaging the phosphinyl group. Double- and triple-click assemblies of azides were accomplished by triazole formations and robust azaylide formation.


 Please wait while we load your content...
Please wait while we load your content...