A metal-free reaction of sulfur dioxide, cyclopropanols and electron-deficient olefins†
Abstract
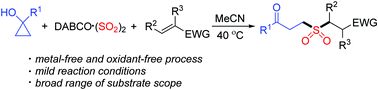
The importance of γ-keto sulfones in medicinal chemistry and organic synthesis is known. An efficient route to γ-keto sulfones via a metal-free reaction of cyclopropanols, sulfur dioxide and electron-deficient olefins is achieved. This reaction proceeds smoothly under mild conditions without the need of catalyst, oxidant or additive. A plausible mechanism is proposed, which occurs through a γ-keto sulfinate intermediate generated in situ from the reaction of cyclopropanol with sulfur dioxide. The γ-keto sulfinate intermediate would be trapped by the electron-deficient olefin, resulting in the formation of γ-keto sulfones. Various functional groups in the cyclopropanols and electron-deficient olefins are compatible in this transformation.


 Please wait while we load your content...
Please wait while we load your content...