A small-molecule probe for monitoring binding to prolyl hydroxylase domain 2 by fluorescence polarisation†
Abstract
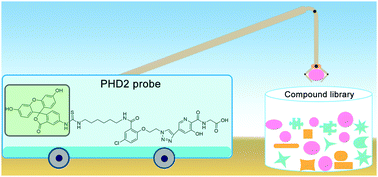
Inhibition of the dioxygen sensing hypoxia-inducible factor prolyl hydroxylases has potential therapeutic benefit for treatment of diseases, including anaemia. We describe the discovery of a small-molecule probe useful for monitoring binding to human prolyl hydroxylase domain 2 (PHD2) via fluorescence polarisation. The assay is suitable for high-throughput screening of PHD inhibitors with both weak and strong affinities, as shown by work with clinically used inhibitors and naturally occurring PHD inhibitors.


 Please wait while we load your content...
Please wait while we load your content...