Metal-encapsulation induces a highly regioselective Bingel–Hirsch reaction of the labile Y@Cs(6)-C82†
Abstract
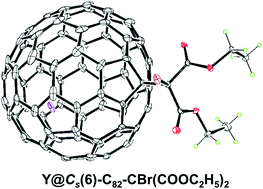
The chemical properties of a prototypical labile mono-EMF, Y@Cs(6)-C82, have been systematically disclosed for the first time via a Bingel–Hirsch reaction. Three mono-adduct isomers, namely, 2a, 2b and 2c out of 44 possibilities for the Y@Cs(6)-C82 cage have been readily isolated, demonstrating surprisingly high regioselectivity. Crystallographic results of 2b unambiguously confirm its molecular structure with a singly bonded bromomalonate group attached onto the Cs(6)-C82 cage. Further computational results rationalize that the high regioselectivity is a consequence of the localization of high spin density and large frontier molecular orbital distribution on the corresponding carbon atoms stemming from the encapsulation of an yttrium atom into the low-symmetry Cs(6)-C82 cage with three-electron transfer from the metal to the cage.


 Please wait while we load your content...
Please wait while we load your content...