Chemical synthesis of α(2,8) octasialosides, the minimum structure of polysialic acids†
Abstract
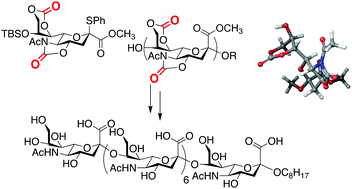
Chemical synthesis of an α(2,8) octasialic acid by using an N-acetyl-5-N,4-O:7-O,9-O-dicarbonyl protected sialyl donor is reported. The glycosyl donor underwent α-selective sialylation at the C8 hydroxyl group to give α(2,8) sialyl oligomers. The resulting oligosaccharides were then deprotected to give the fully deprotected α(2,8) octasialic acid without lacking the N-acetyl groups.


 Please wait while we load your content...
Please wait while we load your content...