Cobalt(ii)-catalyzed hydroarylation of 1,3-diynes and internal alkynes with picolinamides promoted by alcohol†
Abstract
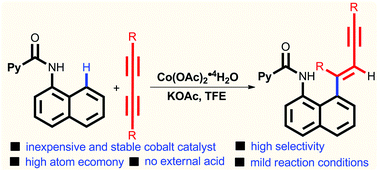
The Co(II)-catalyzed selective C–H alkenylation of picolinamides with 1,3-diynes has been developed. This protocol can be applied to a variety of 1,3-diynes. In addition, both symmetrical and unsymmetrical internal alkynes were well tolerated, affording the corresponding alkenyl arenes. Moreover, control experiments indicated that C–H bond cleavage may be involved in the rate-determining step. Furthermore, a deuterium incorporation product was achieved when deuterated alcohol was employed as the solvent, which suggested that alcohol was essential for the final protonolysis.


 Please wait while we load your content...
Please wait while we load your content...