An in situ approach to functionalize metal–organic frameworks with tertiary aliphatic amino groups†
Abstract
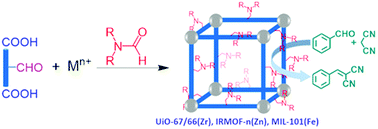
Metal-catalyzed reductive amination of formyl-containing linkers with N,N-dialkylformamide solvents is concomitant with the solvothermal coordination assembly, leading to novel MOFs functionalized with tertiary aliphatic amino groups. This illustrates a novel one-pot strategy to functionalize MOFs through in situ organic transformation. The UiO-66 MOFs partially functionalized with the amino groups are highly active heterogeneous catalysts for Knoevenagel condensation.


 Please wait while we load your content...
Please wait while we load your content...