Desymmetrization of gem-diols via water-assisted organocatalytic enantio- and diastereoselective cycloetherification†
Abstract
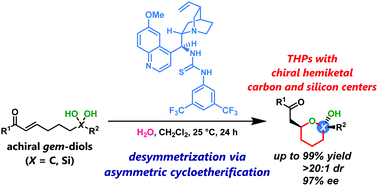
The first desymmetrization of gem-diols forming chiral hemiketal carbons was accomplished via organocatalytic enantio- and diastereoselective cycloetherification, which afforded optically active tetrahydropyrans containing a chiral hemiketal carbon and tetrasubstituted stereocenters bearing synthetically versatile fluorinated groups. The desymmetrization of silanediols was also demonstrated as an asymmetric route to chiral silicon centers.


 Please wait while we load your content...
Please wait while we load your content...