Rhodium-catalyzed cycloisomerization of ester-tethered 1,6-diynes with cyclopropanol moiety leading to tetralone/exocyclic diene hybrid molecules†
Abstract
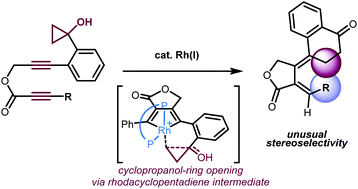
The rhodium-catalyzed cycloisomerization of ester-tethered 1,6-diynes bearing a cyclopropanol moiety produced tetralone/exocyclic diene hybrid molecules with thermodynamically unfavorable alkene geometry. The results of control experiments and density functional theory calculations suggest that the ester tether plays an important role in the efficiency of E/Z isomerization processes.


 Please wait while we load your content...
Please wait while we load your content...