Single-crystal-to-single-crystal transformation and proton conductivity of three hydrogen-bonded organic frameworks†
Abstract
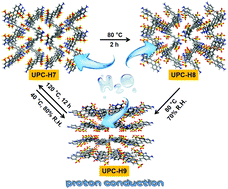
We report the cyclic single-crystal-to-single-crystal transformation of three hydrogen-bonded organic frameworks (HOFs), induced by the change of temperature and humidity, which clearly reveals that the –SO3−and –NH2 groups in UPC-H7 and UPC-H8 facilitate the diffusion of water molecules into their anhydrous structures to form hydrous UPC-H9. Their proton conductivity was studied under different humidity at varying temperature, showing that the proton conductivity is closely related to water molecules entering the crystal structures arising from the hydrogen bonded reorganization in combination with the triaxial single-crystal proton conductivity tests.


 Please wait while we load your content...
Please wait while we load your content...