Conformation change significantly affected the optical and electronic properties of arylsulfonamide-substituted anthraquinones†
Abstract
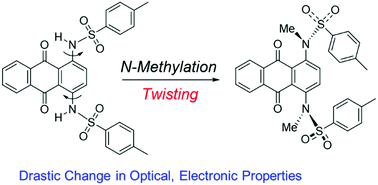
Drastic changes in the optical and electronic properties of arylsulfonamide-substituted anthraquinones were induced by simple N-methylation. N-Methylation at the congested peri-position of the anthraquinone unit induced a drastic conformational change from a coplanar arrangement to an orthogonal relationship between the anthraquinone scaffold and arylsulfonamide substituents. As a result, the contribution of the substituents on the anthraquinone unit was spectroscopically suppressed, as demonstrated by UV-Vis absorption spectra and cyclic voltammograms.


 Please wait while we load your content...
Please wait while we load your content...