A novel, rationally designed lanthanoid chelating tag delivers large paramagnetic structural restraints for biomolecular NMR†
Abstract
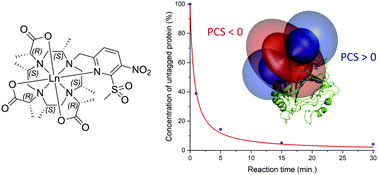
Herein, a novel and rationally designed ortho-substituted pyridine activator is reported that reacts rapidly and selectively with cysteine thiols. It forms reduction-stable conjugates and induces large pseudocontact shifts, residual dipolar couplings and paramagnetic relaxation enhancement on both ubiquitin S57C and human carbonic anhydrase II S50C constructs under physiological conditions.


 Please wait while we load your content...
Please wait while we load your content...