Recent advances in three-component difunctionalization of gem-difluoroalkenes
Abstract
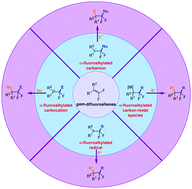
Three-component difunctionalization of alkenes is regarded as one of the most attractive strategies in synthetic chemistry, because it enables the direct synthesis of useful and complex molecules from simple and readily available starting materials in a step-economic manner. gem-Difluoroalkenes, as a class of useful and readily available fluorine-containing raw materials, have been well investigated in two-component coupling reactions. This review focuses on the progress of three-component difunctionalization reactions of gem-difluoroalkenes. On the basis of the intermediates involved in these transformations, these three-component reactions of gem-difluoroalkenes could be categorized into four types: (1) involving an α-fluoroalkylated carbanion, (2) involving an α-fluoroalkylated carbon–metal species, (3) involving an α-fluoroalkylated radical, and (4) involving an α-fluoroalkylated carbocation. The typical transformations, the substrate scope, the reaction mechanisms, and their subsequent applications in the synthesis of bioactive molecules are discussed. Owing to the rapidly increasing interest in this area, we believe that this review will present a timely and comprehensive understanding of the recent progress in three-component difunctionalization reactions of gem-difluoroalkenes.


 Please wait while we load your content...
Please wait while we load your content...