One-dimensional electronic systems: metal-chain complexes and organic conductors†
Abstract
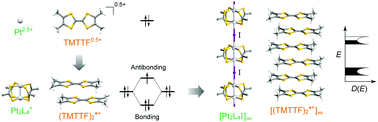
One-dimensional (1D) metal-chain complexes and organic conductors show many similarities as well as striking differences in structural and electronic properties, although constituent elements and orbitals that contribute to charge transfer in these systems are quite different. In this review, we highlighted the structural and electronic properties of neutral MMX-chain complexes (M = Pt2+/3+, X = I−) and tetramethyltetrathiafulvalene-based cation radical salts as typical examples of each group while comparing them with each other. This review primarily aims to construct a coherent body of knowledge of 1D electronic materials that might have been separately investigated. We have proposed future directions for the exploration of new and more advanced electronic materials not only having 1D character, but also residing in the dimensional crossover regime.
- This article is part of the themed collections: ChemComm Community – Dedicated Authors and Functional Coordination Networks


 Please wait while we load your content...
Please wait while we load your content...