FeVO4 porous nanorods for electrochemical nitrogen reduction: contribution of the Fe2c–V2c dimer as a dual electron-donation center†
Abstract
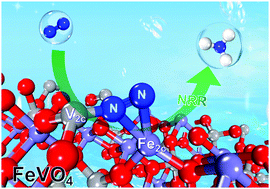
The electrocatalytic N2 reduction reaction (NRR) offers a sustainable route for ambient NH3 production. To ensure a high NRR efficiency, it is critically important to design active electrocatalysts which possess a strong electron-donating capability to activate the stable N![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) N bond and facilitate the protonation process. Herein, inspired by the FeV-cofactor as a catalytic site for biological N2 fixation, we show that the FeVO4 can be a highly efficient and durable NRR catalyst. The developed FeVO4 porous nanorods delivered a favorable combination of both high NH3 production rate (52.8 μg h−1 mg−1) and high faradaic efficiency (15.7%), surpassing those of nearly all the previously reported Fe- and V-based catalysts. Theoretical computations revealed that the high NRR performance of FeVO4 originated from the Fe2c–V2c dimer (2c means two-fold coordinated bond) as a dual electron-donation center to effectively activate the NRR with a low overpotential.
N bond and facilitate the protonation process. Herein, inspired by the FeV-cofactor as a catalytic site for biological N2 fixation, we show that the FeVO4 can be a highly efficient and durable NRR catalyst. The developed FeVO4 porous nanorods delivered a favorable combination of both high NH3 production rate (52.8 μg h−1 mg−1) and high faradaic efficiency (15.7%), surpassing those of nearly all the previously reported Fe- and V-based catalysts. Theoretical computations revealed that the high NRR performance of FeVO4 originated from the Fe2c–V2c dimer (2c means two-fold coordinated bond) as a dual electron-donation center to effectively activate the NRR with a low overpotential.


 Please wait while we load your content...
Please wait while we load your content...