Rapid access to 3-aminoindazoles from nitriles with hydrazines: a strategy to overcome the basicity barrier imparted by hydrazines†
Abstract
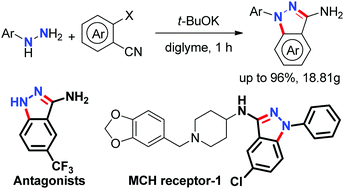
A practical and efficient base mediated synthesis of free 3-aminoindazoles has been developed from the reaction of nitriles with hydrazines, which successfully overcomes the difficulty of using aromatic hydrazines as substrates and allows for the synthesis of a wide range of N-aryl substituted free 3-aminoindazoles in moderate to excellent yields under mild conditions in one-pot. This finding provides a rapid and useful strategy for the synthesis of various functionalized 3-aminoindazole derivatives.


 Please wait while we load your content...
Please wait while we load your content...