Field-based rational design of p300 histone acetyltransferase inhibitor and systematic evaluation as an anti-fibrotic agent†
Abstract
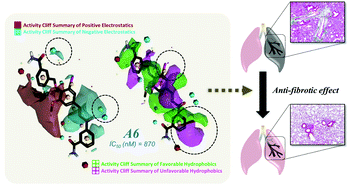
(E)-3-(3-(4-((3-Carbamoylbenzyl)oxy)-3-iodo-5-methoxyphenyl) acryloyl)benzamide (A6) was found to be a potent p300 inhibitor (IC50 = 870 nM) showing a similar binding mode to that of acetyl-CoA, a p300 substrate, and effective anti-fibrotic activity in both TGF-β1-stimulated lung fibroblast cells and bleomycin-induced in vivo lung fibrosis mice.


 Please wait while we load your content...
Please wait while we load your content...