Cu-Catalyzed one-pot synthesis of thiochromeno-quinolinone and thiochromeno-thioflavone via oxidative double hetero Michael addition using in situ generated nucleophiles†
Abstract
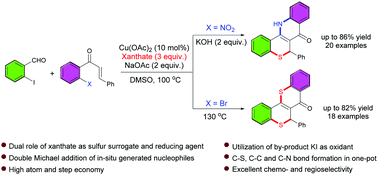
A copper catalyzed three-component synthesis of π-conjugated tetracyclic thiochromeno-quinolinone and thiochromeno-thioflavone was established via oxidative double hetero Michael addition using in situ generated nucleophiles. Xanthate plays a dual role as an odourless sulfur source and a chemoselective reducing agent. The in situ formed iodine plays a crucial role in the oxidation step.


 Please wait while we load your content...
Please wait while we load your content...