Practical and regioselective halo-trifluoromethylthiolation of sulfur ylides†
Abstract
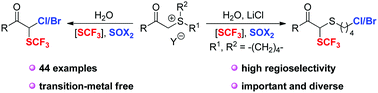
A H2O mediated practical and highly regioselective chloro- and bromo-trifluoromethylthiolation of sulfur ylides is reported using a difunctionalization strategy. In the reaction sequence, sulfur ylides presumably react with an electrophilic trifluoromethylthiolating reagent to generate an α-SCF3 substituted sulfonium salt intermediate, which then undergoes a substitution with nucleophilic halogens.


 Please wait while we load your content...
Please wait while we load your content...