Flow through negatively charged, nanoporous membranes separates Li+ and K+ due to induced electromigration†
Abstract
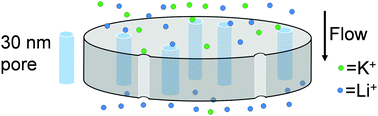
Flow through negatively charged nanopores separates Li+ and K+ with selectivities of up to 70 and Li+ passages from 20% to above 100%. Remarkably, both the Li+/K+ selectivity and Li+ passage initially increase with flow rate, breaking the permeability/selectivity trade-off. Modelling demonstrates that flow through the membranes creates electric fields that retard transport of cations. Selectivity increases with flow rate because the K+ electromigration velocity exceeds its convective velocity, but for Li+ electromigration is weaker than convection. Modelling also shows the importance of controlling concentration polarization. With further work, related separations might provide highly pure Li salts for battery manufacturing.


 Please wait while we load your content...
Please wait while we load your content...
