Palladium-catalyzed ortho-halogen-induced deoxygenative approach of alkyl aryl ketones to 2-vinylbenzoic acids†
Abstract
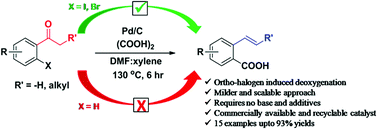
The 2-vinylbenzoic acids have wide applications in the field of polymer chemistry and are key precursors for the synthesis of important bioactive molecules. Herein, an ortho-halogen-induced deoxygenative approach for the generation of 2-vinylbenzoic acids from alkyl aryl ketones by palladium catalysis is discovered and explored. This approach requires no base or stoichiometric additives and can be carried out through a simple one-step process. Furthermore, the present reaction is scalable up to one-gram scale. The commercially available palladium on carbon (5 wt%) was used as a heterogeneous catalyst and showed excellent recyclability (<5 times) without significant loss in catalytic activity. Pleasingly, under our optimized conditions, the alpha alkyl substituted 2-iodoacetophenones exhibit good diastereoselectivity and predominantly (E)-2-vinylbenzoic acids were obtained with good to excellent yields.


 Please wait while we load your content...
Please wait while we load your content...