Evidence and exploitation of dicationic ammonium–nitrilium superelectrophiles: direct synthesis of unsaturated piperidinones†
Abstract
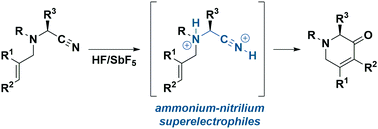
Exploiting superacid activation, the reactivity of aminonitriles was enhanced through the transient formation of highly reactive ammonium–nitrilium superelectrophiles. Demonstrated by using in situ low-temperature NMR experiments and confirmed by X-ray diffraction analysis, these dications can be intramolecularly trapped by non-activated alkenes to generate unsaturated piperidinones, including enantioenriched ones, in a straightforward way.


 Please wait while we load your content...
Please wait while we load your content...