Synthesis of multisubstituted cycloalkenes through carbomagnesiation of strained cycloalkynes†
Abstract
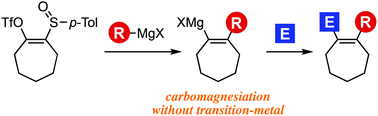
An efficient synthetic method of seven- and six-membered cycloalkenes through the generation of strained cycloalkynes and following carbomagnesiation is described. Further bond formations of the resulting cycloalkenylmagnesium intermediates with a wide variety of electrophiles enabled us to prepare diverse cycloalkene derivatives including benzoxepine analogs having a fully substituted alkene structure.


 Please wait while we load your content...
Please wait while we load your content...