Nickel-catalyzed allyl–allyl coupling reactions between 1,3-dienes and allylboronates†
Abstract
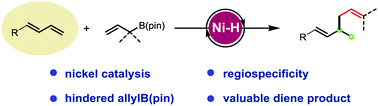
A regiospecific allyl–allyl coupling reaction between 1,3-dienes and allylboronates has been demonstrated under nickel catalysis. Salient features of this method include the earth-abundant metal catalyst, excellent regioselectivity and good functional group tolerance. Notably, even congested allyl substrates can also be applied to this protocol, thus allowing for the rapid preparation of a series of valuable 1,5-dienes.


 Please wait while we load your content...
Please wait while we load your content...