Unprecedented 1,3-tert-butyl migration via the C–N single bond scission of isonitrile: an expedient metal-free route to N-sulfonyl amidines†
Abstract
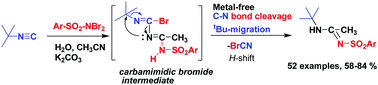
An unprecedented 1,3-migration of the tert-butyl group was observed while reacting tert-butyl isonitrile with N,N-dibromoaryl sulfonamides and nitrile. The reaction involves the simultaneous C–N single bond scission of isonitrile and the migration of the tert-alkyl group to the adjacent unsaturated nitrogen centre of the nitrile precursor, which eventually results in the formation of N-sulfonyl amidine. This method constitutes a new route for sulfonyl amidine, which does not rely on transition metals. The protocol exhibits a significant advantage in terms of substrate scope and additionally offers an easy synthesis route to guanidine molecules.


 Please wait while we load your content...
Please wait while we load your content...