Consecutive borylcupration/C–C coupling of γ-alkenyl aldehydes towards diastereoselective 2-(borylmethyl)cycloalkanols†‡
Abstract
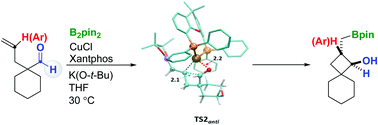
Copper(I) catalyzes the borylative cyclization of γ-alkenyl aldehydes through chemo- and regioselective addition of Cu–B to C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C and concomitant intramolecular 1,2-addition of Cu–C on C
C and concomitant intramolecular 1,2-addition of Cu–C on C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O. The products are formed in an exclusive diastereoselective manner and computational analysis identifies the key points for the observed chemo- and diastereoselectivity.
O. The products are formed in an exclusive diastereoselective manner and computational analysis identifies the key points for the observed chemo- and diastereoselectivity.


 Please wait while we load your content...
Please wait while we load your content...