Electronic structure inspired a highly robust electrocatalyst for the oxygen-evolution reaction†
Abstract
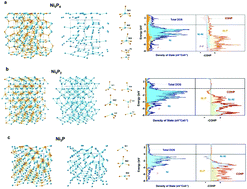
We demonstrated that the electronic-band structure holds the key to electrocatalytic durability towards the oxygen-evolution reaction (OER). Density functional theory (DFT) revealed the characteristic of Ni–Ni bonding interactions within Ni5P4, Ni5P2 and Ni3P were different and could influence their phase stabilities during the OER. Ni5P2 and Ni3P exhibited very robust OER performances at high current density (>350 mA cm−2) over 12 h whereas, for Ni5P4, obvious deterioration was observed. In situ/ex situ X-ray near-edge structure, high-resolution transmission electron microscopy, and X-ray photoelectron spectroscopy indicated the phase stability of Ni5P4, Ni5P2 and Ni3P behaved differently during the OER. These materials transformed to Ni oxyhydroxide but the process for Ni5P2 and Ni3P was much slower even under high anodic potential 1.6 V (V vs. RHE). These results supported the theoretical prediction and provide a refreshing viewpoint for designing reliable electrocatalysts for the OER.


 Please wait while we load your content...
Please wait while we load your content...